Amendments have been made to the law on the circulation of medicines with a view to harmonising it with EAEU legislation
Pepeliaev Group advises that Federal Law No. 1-FZFederal Law No. 1-FZ dated 30 January 2024 “On amending the Federal Law 'On the circulation of medicines’ and articles 1 and 4 of the Federal Law ‘On amending the Federal Law ‘On the circulation of medicines’ and the Federal Law ‘On amending the Federal Law 'On the circulation of medicines’”.
was adopted on 30 January 2024 to amend Law No. 61-FZ “On the circulation of medicines”.Federal Law No. 61-FZ “On the circulation of medicines” dated 12 April 2010 (the ‘Law on the circulation of medicines’, or ‘FZ-61’).
The amendments are designed to harmonise Russian legislation with the unified requirements for the circulation of medicines on the EAEU market and to improve current regulation.
The law comes into effect starting from the date on which it was published, i.e. 30 January 2024, except for certain provisions which will come into force in three stages as follows: on 1 September 2024, on 1 January 2025, and on 1 January 2026.
How it used to be
The draft law was submitted to the State Duma in August 2023 and immediately came into the focus of the pharmaceutical industry as it proposed a number of significant changes. When the draft law was considered during its second and third readings, amendments were made to it based on various recommendations and opinions. Some of the amendments which were proposed were originally slated for elimination.
Exclusivity of data
One of the key issues addressed in the draft law was the legal regulation of the exclusivity of data from clinical trials. The final version of the law has preserved provisions concerning the exclusivity of data, but these will now be set out in parts 51–53 of article 13 of the Law on the circulation of medicines.
Consequently, current regulation of exclusivity of data will be preserved looking as follows:

Comment
The moving of the above provisions relating to the protection of data relating to pre-clinical and clinical trials to a different place in the text of the law does not, in our opinion, solve the problem that has manifested itself in practice of the inconsistency between the provisions of national legislation and of EAEU legislation, which does not have similar provisions.
There are disputes in practice which have been fuelled by such inconsistencyResolution No. F05-21974/2023 of the Commercial Court for the Moscow Circuit dated 26 September 2023.
Currently, the case is pending before the Russian Supreme Court’s Judicial Board for Economic Disputes (see the case card).
: courts rule that no refusal may be issued for a generic medicine to be registered before four years expire after the reference medicine has been registered because this contradicts the provisions of Decision No. 78 of the Eurasian Economic CommissionDecision No. 78 of the Board of the Eurasian Economic Commission “On the rules for the registration and expert examination of medicines for medical application” dated 3 November 2016.
.
Terminology
The terminology of the Law on the circulation of medicines has been extended. There is a new definition of a high-tech medicine, which is understood to mean a gene-therapy medicine or a human cell-based medicinal product or a tissue-engineered product.
Electronic public services
Starting from 1 January 2025, public services which relate to the state registration of medicines for human or animal use will be provided electronically with the use of the Federal Government's “Unified Portal of Government and Municipal Services (Functions)” Information System.
Instead of marketing authorisations on paper, the fact of registration will be evidenced by an entry in the register and the holder, at their request, will obtain an excerpt certified by the relevant competent authority.
Launching on the market
Considerable amendments have been made to the procedure for launching medicines on the market.
It will now also apply to pharmaceutical substances.
The remaining amendments are, from our perspective, designed to reduce the volume of regulatory requirements and expenses involving launching medicines on the market.
The main amendments are shown on the diagram below.
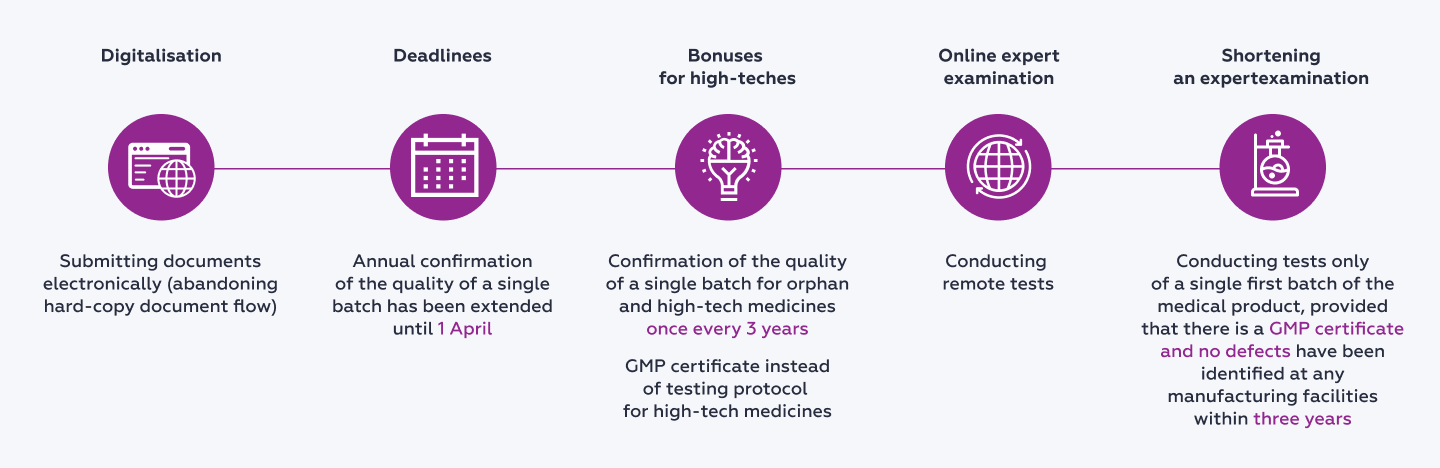
Documents necessary to obtain permissions to place medicines on the market will now be provided electronically. The decision has been made to fully abandon any hard-copy flow of documentation.
The deadlines have been extended for test protocols to be submitted annually for one batch of a medicine which has been launched on the market. Now, such protocols are to be submitted to the Russian Federal Service for Surveillance in Healthcare (in Russian, abbreviated to ‘Roszdravnadzor’) by 1 April rather than by 1 February, as the current legal provisions stipulate.
The procedure has been simplified for launching new medicines on the market. A manufacturer (importer) has the right to submit a test protocol from a federal laboratory only with respect to one batch (consignment) of medicine which has been manufactured or imported provided that two additional conditions have been met:
-
it submits, together with the above, a GMP certificate for Russia or a GMP certificate for the EAEU of the manufacturing facility where the medicine was produced;
-
not a single case of a defect has been recorded during the previous three years at any of the manufacturing facilities of the manufacturer.
The specific aspects of orphan and high-tech medicines, including for individual use
Certain relief has been made available to manufacturers of orphan and high-tech medicines which are derived from a biological material of a particular person which are designed to be administered to the same person. For the first batches to be put into circulation, there is no need now to submit a test protocol regarding whether a batch or consignment of a medical product corresponds to the quality indicators at the federal laboratory.
A test protocol of the first batch (consignment) of high-tech products for individual use may be obtained via remote tests being conducted and be submitted to Roszdravnadzor within first three months after the medicine has been put on the market (part 43 of article 52.1 of FZ-61). A similar approach to conducting expert examinations has been already successfully tested by the regulator in 2022 against the backdrop of increasing sanctions pressureThe Guidelines for compliance with mandatory requirements of legislation in the sphere of the circulation of medicines relating to the specific aspects of how medicines for human use should be launched on the market (Section VII of the Russian Government's Resolution No. 593 dated 5 April 2022) (as approved by the Federal Service for Surveillance in Healthcare on 14 November 2022).
.
Instead of annually submitting test protocols in relation to high-tech medicines their manufacturers may submit a GMP certificate for Russia or a GMP certificate for the EAEU of the manufacturing facility where such medicines were produced.
The procedure for determining the possibility to consider a medicine an orphan medicine may be conducted with regard to a medicine which has already been registered or which has not. The State Register of Medicines (SRM) should contain information about whether the medicine is an orphan medicine.
Information will be included in the SRM regarding whether the medicine is orphan, immunobiological, radiopharmaceutical, homeopathic, biological or high-tech medicine which has for the first time been registered in Russia according to the national rules or the rules of the Union.
Moreover, the obligation may be excluded for manufacturers of high-tech medicines for human use to label the packaging with means of identification if a medicine is manufactured in small quantities, consists of one or several doses and has a short shelf life (up to 90 calendar days), or a temperature storage and transportation regime of 60 °С and below.
Cancellation of state registration and suspension of use
Starting from 1 January 2026, the provision of article 32(8) of FZ-61 regarding the cancellation of the state registration of a medicine where such medicine has not been used in Russia for 3 years and more will no longer apply with respect to medicines for human use but will be preserved for veterinary products.
Comment
Originally, it was proposed that the procedure for the suspension of a registration be excluded owing to the fact that there is no such procedure in the legislation of the Union. However, it was decided to keep this provision in the final version of the law. The question remains of how this mechanism will be applied and whether courts confirm that it can be used.
Medicines in foreign packaging
Starting from 1 September 2024, it will be permitted, on a continuing basis, to import orphan and high-tech medicines within 12 months after such medicines have been registered in foreign packaging (currently, such imports are permitted until the end of 2024). However, this provision does not mean any changes in relation to the current rules concerning parallel import. The list of conditions for a specific batch of medicines to be imported will be extended with the purposes of developing medicines and conducting scientific and other research.
What to think about and what to do
Developers and manufacturers of medicines will have to adjust their internal procedures for preparing registration dossiers and packages of documents to amend them in view of the anticipated transfer to submitting documents electronically to the registration authority.
Manufacturers of orphan and high-tech medicines can also be advised to conduct an audit of business processes and increase control over the quality of production to be able to tap into the advantages of launching their products on the market.
Help from your adviser
Pepeliaev Group’s lawyers are ready to provide necessary advisory support in relation to matters concerning the circulation of medicines, including those which are attributed to ongoing changes in regulation.
Translated by the Translation Department of Pepeliaev Group.
