

|
||
Pepeliaev Group advises that, starting from 1 September 2023, it will be mandatory to label with means of identification biologically active supplements, skin sanitisers, individual types of medical products, and other goods.
The Russian Government has approved, by adopting four decrees, the start of mandatory labelling of biologically active supplements, individual types of medical products, sanitisers and wheelchairs.
|
|
Biologically active supplements Decree No. 886 of the Russian Government dated 31 May 2023 |
|
Having a certificate of state registration and corresponding to the following commodity classification codes:
1) Commodity Classification for Foreign Trade: 1204 00 900 0, 1208 90 000 0, 1210 20 900 0, 2) Russian National Classification of Products 2: 01.11.91.120, 01.28.20, 03.11.63, 10.13.15.193, 10.13.15.194, 10.13.15.199, 10.41.12.110, 10.41.29, 10.41.42, 10.41.59, 10.41.60, 10.42.10, 10.61.33, 10.62.13.111, 10.62.13.122, 10.62.13.130, 10.62.13.190, 10.82.22.121, 10.82.22.122, 10.82.22.190, 10.82.23.121, 10.83.12.120, 10.85.19, 10.89.15, 10.89.19.140, 10.89.19.210, 10.89.19.290, 11.07.19.190, 21.10.20, 21.10.51, 21.10.60.193, 21.10.60.194, 21.20.10.255 |
|
|
|
Wheelchairs Decree No. 885 of the Russian Government dated 31 May 2023 Commodity Classification for Foreign Trade: 8713 10 0000, 8713 90 0000; Russian National Classification of Products 2: 30.92.20.000, 32.50.50.190, 30.99.10.190; 20.42.15 |
|
|
Sanitisers Decree No. 870 of the Russian Goverment dated 30/05/2023 1) Perfumes and cosmetics with an antimicrobic effect (Commodity Classification for Foreign Trade: 3304 99 000 0; Russian National Classification of Products 2: 20.42.15); 2) skin sanitisers - disinfecting agents (Commodity Classification for Foreign Trade: 3808 94 800 0; Russian National Classification of Products 2: 20.20.14.000) |
|
|
Individual types of medical products Decree No. 894 of the Russian Government dated 31 May 2023 1) disinfectors - air cleaners; orthopaedic shoes and corrective inner soles, half socks (Commodity Classification for Foreign Trade: 8421 39 200 8, 8421 39 800 6, 8539 49 000 0, 9018 20 000 0, 9021 10 100 0; Russian National Classification of Products 2: 28.25.14.110, 32.50.22.150, 32.50.22.151, 32.50.22.152, 32.50.22.155, 32.50.22.156, 32.50.22.157, 32.50.50.190; codes of types of medical product according to the commodity classification: 131980, 152690, 152700, 182750, 209360, 250220, 250230, 250250, 250260, 292620, 320560, 336330, 343610, 375930); 2) hearing aids; coronary stents; computed tomography scanners; pads and diapers for adults (Commodity Classification for Foreign Trade: 9021 40 000 0, 9021 90 900 1, 9022 12 000 0, 9022 13 000 0, 9022 14 000 0, 9022 19 000 0, 9619 00 890; Russian National Classification of Products 2: 17.22.12.130, 26.60.11.111, 26.60.11.113, 26.60.11.119, 26.60.14.120, 32.50.22.190, 32.50.22.195; codes of types of medical product according to the commodity classification: 113850, 126750, 135190, 135820, 142570, 155760, 155800, 155820, 173110, 202800, 202810, 204370, 210000, 218190, 228560, 233730, 233860, 233900, 273880, 280360, 280730, 282030, 302870, 320550, 331320, 331330, 331830, 343410, 343540, 343580, 356150). |
Now, every participant in the circulation of the above goods will have to register with the Goods Labelling information monitoring system hosted at the Chestny ZNAK portal (the “IS”).
Applications for registration in the IS are to be filed starting from 1 September 2023. This should be done within seven calendar days after the necessity arises for the participant in the circulation of the goods to act in connection with introducing such goods into circulation, and/or the process of circulation, and/or withdrawing them from circulation. Within 15 calendar days after registration in the system, a participant should prepare their own software for information exchange with the IS and file an application for testing with the operator.
It is important to bear in mind that the labelling codes are provided against payment, except for a case where a participant in the circulation had started labelling the goods before mandatory labelling was introduced; in such case, the codes are provided free of charge. The codes should also be paid for if they have not been transformed into means of identification and no report was submitted to the IS before 30 September 2023 about such means having been applied.
The labelling codes may be obtained via entering into an agreement between the operator and a participant in the circulation of goods. At present, the template forms of an agreement for services aimed at providing labelling codes as developed by the Russian Ministry of Industry and Trade are undergoing an anti-corruption review. We assume that they will be approved in one version or another in the nearest future.
Starting from 1 October 2023, the manufacturers and importers of these pharmaceutical products are obliged to apply means of identification to the package of the above types of products.
General requirements for participants in circulation to connect to the IS:
enhanced qualified digital signature (EQDS);
software and hardware ensuring that electronic documents can be signed with an EQDS;
remote access to an emission recorder located in the infrastructure of the monitoring information system;
a signed agreement with an operator of electronic document management;
Requirements for retail sellers who use cash register equipment:
software and/or technical means for the recognition of means of identification, which are linked to the cash register equipment;
An agreement entered into with an entity which has obtained consent to the processing of fiscal data as part of its use of cash register equipment, the subject matter of such agreement being the processing and transferring to the information monitoring system of data relating to the circulation of goods.
It is important to note that participants should record the goods being transferred via the system of electronic document management, with universal transfer documents (“UTDs”) being issued. In this connection, the following particular aspects should be taken into account of the agreement which is entered into between the operator and a participant of the circulation of goods:
the UTDs, signed by both parties, between the participant in the circulation of goods and the operator of the electronic document management are transferred in real time;
the date when the obligation is discharged of a participant in the circulation of goods to submit data to the IS is deemed to be the date when the UTDs are received;
the operator of the electronic document management is liable for the accuracy of the data contained in the UTDs as well as for the transfer documents, presented by the participant in the circulation of goods, to be continually and timely submitted to the IS.
The timeframes for introducing labelling vary depending on the type of goods. The start of the mandatory labelling does not mean that unlabelled goods are prohibited from circulating. The system of mandatory labelling and tracking of goods is being introduced in several stages: first, registration with the operator, then the codes are applied to the goods and the information is entered into the system, then the circulation of unlabelled goods is prohibited.
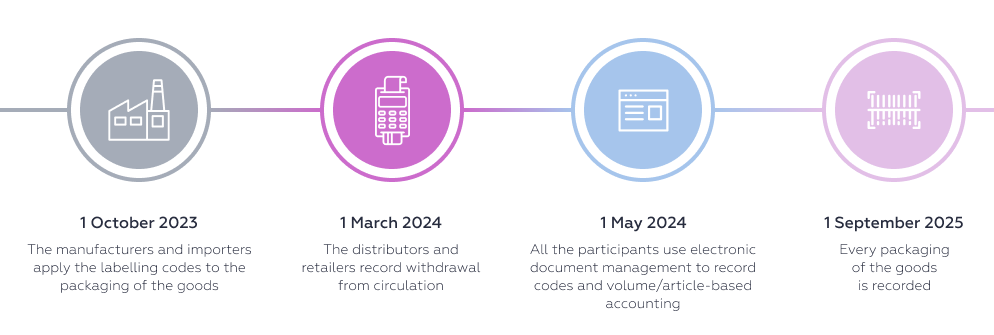
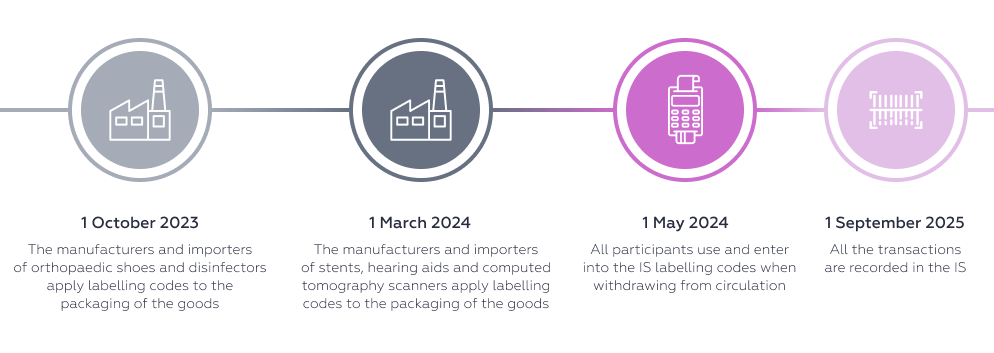
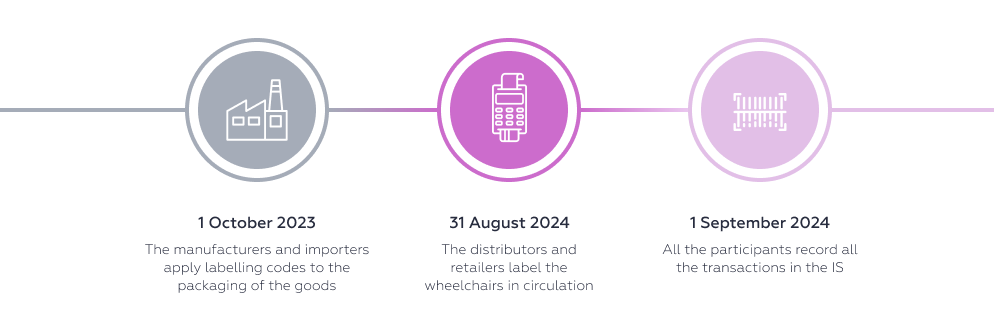
After the switch to all transactions with individual codes being recorded in the IS, the circulation of unlabelled goods will be prohibited. In other words, starting from 1 September 2025 it will be prohibited to sell or otherwise withdraw from circulation biologically active supplements, skin sanitisers and individual types of medical products without entering the labelling codes into the IS. For wheelchairs, the deadline is earlier: 1 September 2024.
Effective Russian legislation provides for administrative and criminal liability for the absence of labelling, non-compliance with the procedure for applying it, and non-submission of information to the IS.
|
Administrative liability |
Criminal liability
|
|
|
Article 15.12.(2) of the Code of Administrative Offences |
Article 15.12.1. of the Code of Administrative Offences |
Articles 171.1.(3) and 171.1.(4) of the Criminal Code |
|
Manufacturing, placing into circulation or selling unlabelled goods, as well as violating the procedure for applying such labels and/or information. |
Failing to provide information, and/or violating the procedure and timeframes for providing information, or providing incomplete and/or inaccurate information to the operator of a state-owned IS monitoring the circulation of goods to be mandatorily labelled with means of identification. |
Manufacturing, purchasing, storing, transporting or selling goods and products without labelling and/or information being applied as stipulated by Russian legislation: on a large scale or especially large scale[1]. |
|
Entities will have a fine imposed of: between RUB 50,000 and RUB 300,000, with the items involved in the administrative offence being seized. |
A warning or a fine 2) for an entity: ranging from RUB 50,000 to 100,000. |
On a large scale: 1) a fine of up to RUB 400,000, or equal to the convicted person's salary or other income for a period of 2 years; 2) or compulsory labour for a period of up to 3 years; 3) or imprisonment for up to 3 years, with a fine of up to RUB 80[A.1] ,000 or equal to the convicted person's salary or other income for a period of 6 months. On an extremely large scale: 1) a fine of up to RUB 700,000, or equal to the convicted person's salary or other income for a period of 3 years; 2) or compulsory labour for a period of up to 5 years; 3) or imprisonment for up to 6 years, with or without a fine of up to RUB 1,000,000 or equal to the convicted person's salary or other income for a period of 5 years. |
In order to mitigate risks, it is advisable to set out in agreements with contractors the relevant undertakings and warranties that the mandatory requirements for entering information about labelling products will be complied with, as well as the liability for such requirements being violated, including the compensation of losses related to the participant being held liable.
Internal regulations should be put in place governing the procedure for providing information to the IS as well as the appointment of persons responsible for working with the system, and the necessary updates/adjustments should be made in other relevant internal regulations, job descriptions and other instruments which are in effect in the company.
The new process should be set out within the framework of the risk management system in the company.
Taking into account the ongoing changes, Pepeliaev Group’s lawyers are ready to provide the necessary legal support on issues directly or indirectly related to the mandatory labelling of goods.
[1] ‘On a large scale’ refers to the value of unlabelled food products exceeding RUB 400,000, whereas ‘on an especially large scale’ refers to a value exceeding RUB 1.5 million.